
In vitro diagnostics (IVDs) are a tiny fraction of overall healthcare costs, but they play an outsized role in patient diagnosis and decisions. While medical devices can cause physical harm to a patient or user through direct impact, IVDs can also inflict indirect harm through improper diagnosis or patient care caused by incorrect results. Regulators in Europe realized that a much wider product offering of IVDs needed to be held to the same standard as medical devices with regard to clinical evidence and performance. With that in mind, the In Vitro Diagnostic Regulation (IVDR 2017/746) was generated with a strong emphasis on performance evaluation.
Your medical device colleagues have been doing clinical evaluation reports for years, but for many IVD professionals (perhaps you) with regulatory compliance oversight of IVDR Class B, C, and D devices, it can be pretty daunting to know where to begin this process.
Don’t despair. There are thousands of people just like you blankly staring at their computer screens, reviewing the IVDR in minute detail along with an associated tangle of guidance documents and standards to understand performance evaluation requirements.
Let’s fix this or at least make some sense of it.
First, you need to arm yourself with the right information. Create a folder on your desktop and grab the PDF documents listed below. There are, of course, many other relevant documents, but these are a great starting point. You will find references to other documents inside the GHTF SG5 documents noted below.
One of the most important building blocks of an IVD performance evaluation report (PER) is clinical evidence. Your clinical evidence is used to support marketing and labeling on the device, including claims about performance. It is one component of your technical documentation that complements your design verification and validation, risk analysis, and other activities. Clinical evidence can come from several pre- and postmarket sources, such as:
Take a look at SG5 N7:2012 and see page 9. It provides a useful flowchart showing the different stages of the clinical evidence process.
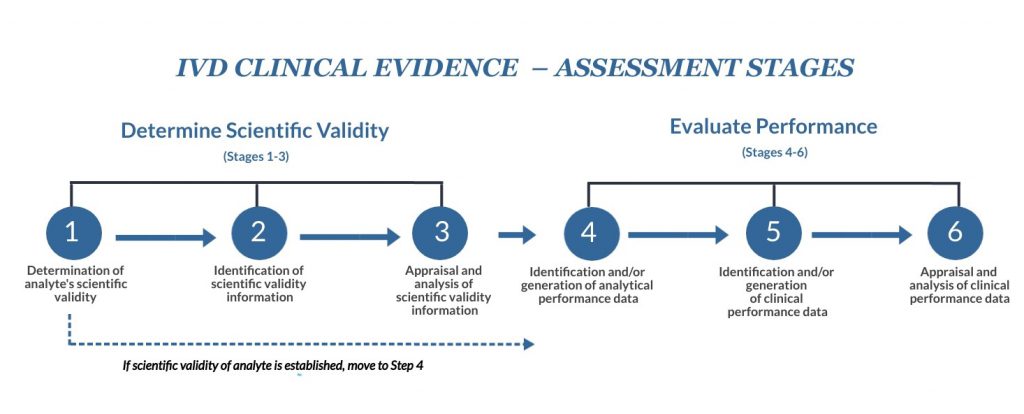
In simple terms, scientific validity is the link between the analyte and the clinical condition/physiological state in question. These first three phases focus on collecting and evaluating what the device does, how it functions, and what disease state is being examined.
If a specific clinical condition or physiological state is well associated with an analyte, then not much needs to be done here. Just add a brief note stating this in a scientific validity report that is part of your clinical evidence and skip to Stage 4. However, consider Stages 2 and 3 just to confirm that the scientific validity of the analyte does not need further supporting evidence.
You have several options here. SG5 N7:2012 guidance notes that you can:
This is where you look at all that data and select information based on pros and cons. Is the information relevant? Is it high quality? Is there a clear association between the clinical condition/physiological state and the analyte? Your analysis should aim to evaluate all of the information based on quality and significance, inclusive of references, justifications, and conclusions. A summary of your analysis of scientific validity becomes part of your clinical evidence report.
During these stages, you will be taking all the relevant data collected in Stages 1-3 and analyzing it to demonstrate the analytical and clinical (if applicable) performance of your IVD for the stated intended use. The requirements of this analysis correlate with the type of device.
1 – Established and standardized tests.
Examples: Liver, kidney, and metabolic markers; hormones; blood gases
2 – Established and non-standardized tests.
Examples: Tests for infectious diseases, as well as for cardiac, tumor, and cell markers
3 – Novel tests.
Examples: Newly identified cardiac and tumor markers, pathogens, emerging infectious diseases (COVID-19, etc.), and pharmacogenomics
All IVD device manufacturers are expected to perform analytical performance studies that, most often, require human specimens. Analytical studies examine the ability of an IVD to detect or measure a particular analyte. These are technical performance tests intended to measure factors such as accuracy, analytical sensitivity/specificity, linearity, cut-off, carryover, and more. These analytical performance studies are often performed internally in the company using banked specimens, retrospective samples or testing, or even nonclinical forms of analysis such as interfering substances or stability.
The second part of this process involves clinical performance evaluation, which shows the relationship between the test results and patient clinical condition. Generally, the performance of an IVD can be measured against diagnostic specificity and sensitivity, predictive value, likelihood ratio, and expected values in baseline and affected populations.
Sections 7.1 and 7.2 of SG5 N7:2012 contain more information about how these studies should be approached as they relate to the three categories of tests noted above. Compilation of this information would be contained in an analytical performance report, which is another element supporting clinical evidence.
Clinical performance looks at the ability of an IVD to produce results that are correlated with a specific clinical condition or physiological state. There are three potential sources of clinical performance data:
Section 7.2.1 of SG5 N7:2012 provides more information on each of the three sources noted above.
As we mentioned earlier, clinical performance data needs to be relevant to the IVD in question and its intended use, and evaluated based on its clinical benefits and limitations. This analysis is intended to collectively look at all of that data and weigh which data is most relevant and important. As you conduct literature searches, one thing you’ll want to watch out for is repeated publications on the same group of patients by the same authors. This can result in overweighting of the data. As with your evaluation of analytical performance, you’ll want to compile all of this in a report that includes references, justifications, and conclusions. The clinical performance report is the third report that supports clinical evidence, which becomes part of the overall performance evaluation process.
The compilation of all your research on scientific validity and analytical/clinical performance will come together in your performance evaluation report. The idea here is to demonstrate that your device meets the relevant general safety and performance requirements outlined in the IVDR Annex I. Your report should outline:
The level of detail you put into this report is dependent on the risk class of your device, and the final report for a Class D device will likely be significantly more detailed than one prepared for a Class B device.
Oriel STAT A MATRIX has a number of ways we can assist you with IVDR preparation. Consider this IVDR training course or, for more specific support, our IVD consulting team is ready to help in any way you need.

US OfficeWashington DC
EU OfficeCork, Ireland



UNITED STATES
1055 Thomas Jefferson St. NW
Suite 304
Washington, DC 20007
Phone: 1.800.472.6477
EUROPE
4 Emmet House, Barrack Square
Ballincollig
Cork, Ireland
Phone: +353 21 212 8530