
Blogs. Social media. Online forums. Product reviews. While postmarket clinical follow-up (PMCF) studies, user surveys, clinical registry studies, electronic health records, and insurance claims data are all important sources of proactive data, most complaints will never cross your desk. Instead, users will often take to online forums and social media to voice their concerns. These potential sources of postmarket surveillance (PMS) data are probably not high on your priority list. After all, trying to extract relevant and actionable PMS data posted online by users may seem like far more work than it is worth.
Yet, there is now a need to get more proactive about collecting PMS data, driven in part by regulators. The new European Medical Device Regulation (MDR), in particular, hammers this point home, emphasizing the importance of responding to issues faster (or preventing them) before they cause more harm.
Let us look at the potential trove of data at your fingertips and how you might find, assess, and trend those little golden nuggets you find online.
By virtue of sheer volume, it is true there are a lot more user data online for consumer medical devices. However, there are also a wide variety of professional online forums dedicated to doctors, dentists, nurses, and clinicians who use medical devices every day. Some potential sources of PMS data include:
1 – Condition-specific online forums
2 – Healthcare professional discussion forums
3 – Product reviews
Let us look at each of these and what to expect.
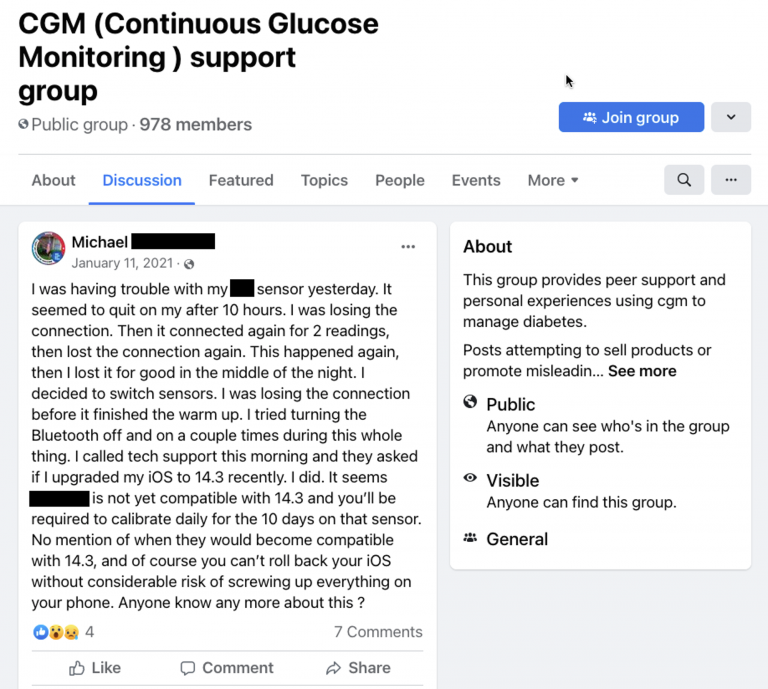
One example of the many medical device related discussion groups on Facebook.
Keep in mind that social media brings out the dramatic nature in people. Situations are sometimes exaggerated for dramatic effect but still need to be taken seriously.
What if you sell a professional-use device? Is there still information to be found online’ Yes, indeed! Sermo is one such example of an online forum where hundreds of thousands of clinicians and doctors worldwide converge to discuss treatments and options. Reddit and all nurses are also potential useful sites for information from doctors, nurses, and other healthcare professionals.
If you sell a consumer device, you may be able to extract insights from product reviews. Comments from users can be useful for spotting issues with instructions for use (IFUs), as users will often express frustration online when they cannot figure out how to use a product they just purchased. See examples below.
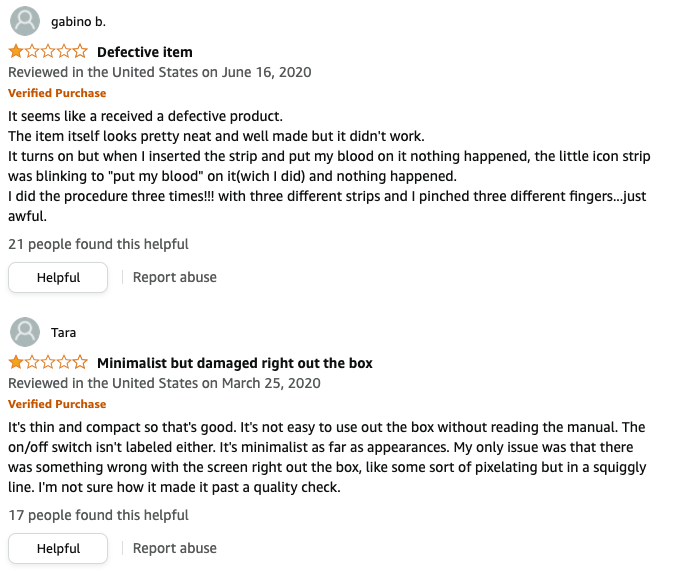
Problems with IFUs can be uncovered by reading reviews.
Do not just look at the big sites such as Amazon. You might also find consumer product reviews such as the ones shown below.
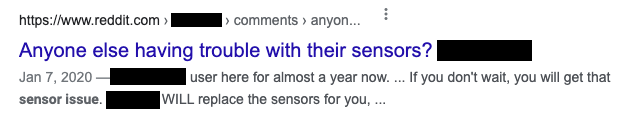

Some of these are from users, while others may be from websites dedicated to reviewing products.
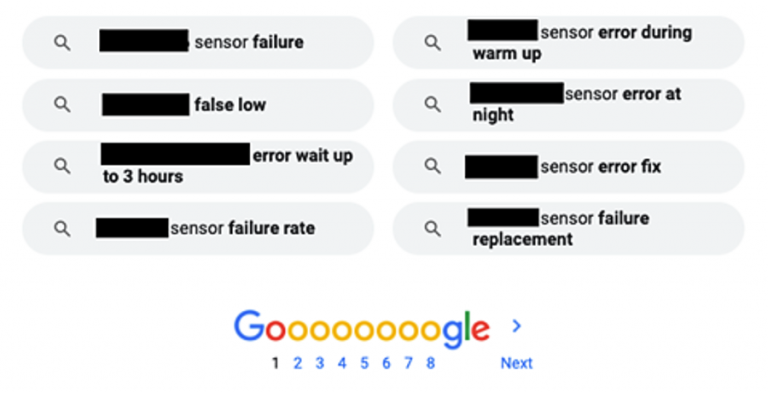
Let’s face it, you have far different needs than your colleagues in marketing. While they are interested in spreading the love for your product, you look through the lens of product safety. For this reason, we recommend that the regulatory department set up its own social media listening tool set. What is that, you ask? These are online tools that scour all corners of the internet looking for mentions of your company, brands, or competitors! They are essential for sifting through troves of online reviews and comments from social media sites, blogs, forums, and shopping sites. Some affordable online listening tools include:
Ask your friends in marketing which social media listening tools they use and prepare to get a surprised response that you even know these tools exist. If they use them, they can help set up yours.
Just because you find some interesting information on social media that does not mean you will be able to gather more information from the user about that specific situation. You are generally not going to be able to reach out to users and investigate complaints. Aside from Facebook, social media users on YouTube, Instagram, and other platforms are identified only by usernames. This can actually be an advantage. While users on Facebook may be reluctant to share device-related experiences with friends and family, that is not the case at all when people post online under pseudonyms to random strangers. While some platforms might allow you to reach out to a user via direct messaging on the app others will not.

One last thought. It is all well and good to gather all of this information online, but many questions remain:
Here are a few tips.
Struggling to build out a robust and compliant PMS program? Oriel STAT A MATRIX offers a dedicated medical device postmarket surveillance training class that will help you stay on top of regulatory requirements and complement your ongoing risk management efforts.

US OfficeWashington DC
EU OfficeCork, Ireland



UNITED STATES
1055 Thomas Jefferson St. NW
Suite 304
Washington, DC 20007
Phone: 1.800.472.6477
EUROPE
4 Emmet House, Barrack Square
Ballincollig
Cork, Ireland
Phone: +353 21 212 8530