Understanding Medical Device Regulatory Training Requirements and Determining Who Needs to Know What
- Oriel STAT A MATRIX
- 0
- on Apr 11, 2019
Who needs to be trained’
What do they need to be trained on’
Does everyone need to be trained on everything’
How often do people need to be trained’
All training and RA/QA managers need to ensure that their organizations meet FDA and international regulatory requirements for training.However, answering the questions above can present a challenge.
Although the FDA regulations and ISO 13485:2016 require training, they do not prescribe training for specific roles. This makes many training managers wonder what exactly constitutes training and how to document it. To better understand regulatory training requirements, lets first compare the relevant sections of the FDA Quality System Regulation 820.25(b) and Clause 6.2 of ISO 13485:2016.

Determining What FDA and ISO 13485 Training Everyone Must Receive and Should Receive
As you can see, the FDA Quality System Regulation (QSR) and ISO 13485 intentionally sidestep the specifics of how to train and how much training is appropriate. Given the vast differences in training needs between a medical device startup and a multinational manufacturer, that makes sense. Generally, small companies that can fit everyone into a single conference room will train everyone on everything, but that becomes impractical and unnecessary as companies grow.
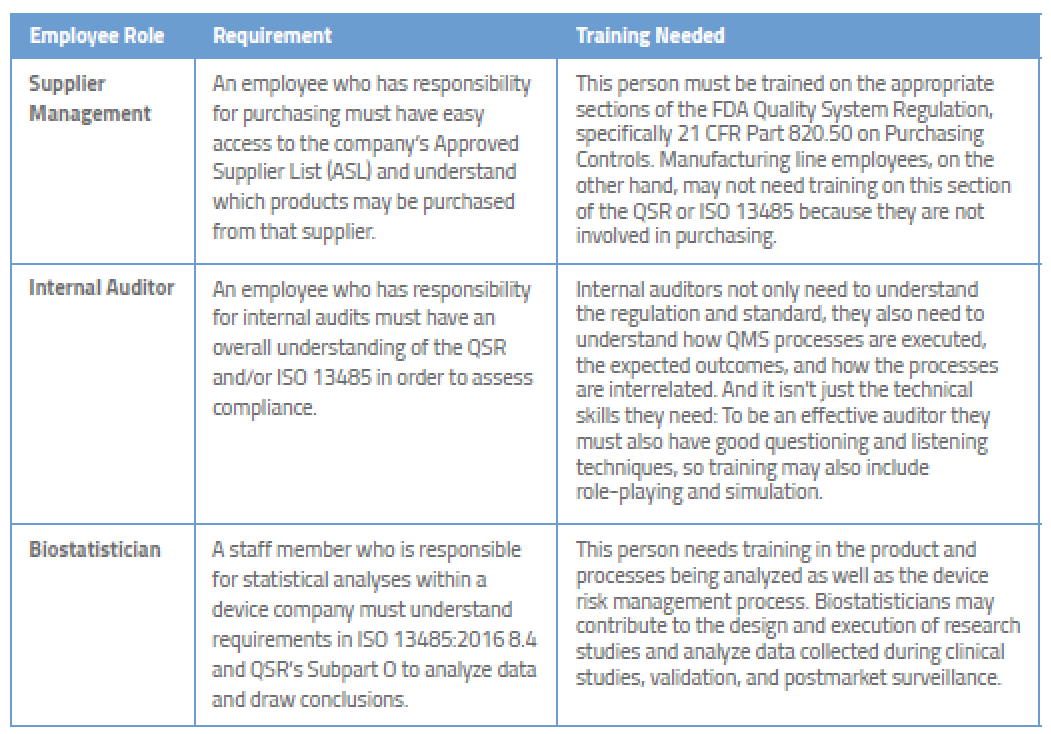
FDA QSR 820.25(b) stipulates that employees must be trained to adequately perform their assigned responsibilities. That language is key, because FDA is saying that you do not need to train everyone on everything you just need to train people on the basics and sections relevant to their role. Here are some examples:
Examples of Required Medical Device Training for Employees
As the company grows and adds ever-more specific standard operating procedures (SOPs) and work instructions, a narrower group of employees must be trained in those requirements. For instance, it is probably a waste of time to train marketing staff on specific assembly procedures, unless you simply want them to have a better grasp of what goes into making your products.
On the other hand, all company employees need to recognize a complaint and know how to escalate it within the organization. For example, you may hand someone a business card at a conference and that person casually mentions a relative who had some issues with your product. Is that a complaint you need to do something about’ Very likely it is.
Other aspects of the regulation and standard on which all employees need at least awareness training include:
- Quality management system (QMS) and policies
- Document control
- The nature of your devices and business
Evaluating Employees Education, Background, and Experience to Determine Competence
Competence is another issue that flummoxes many managers. If your Purchasing Manager has a high school education but 10 years of real-world experience in purchasing, is that adequate for this role’ Because the FDA QSR and ISO 13485 do not define competency, it is up to managers to convince an auditor that a specific person is competent to fill the role. For most staff, managers would typically define the skills required in the job posting and/or in the job description. Thus, you are in essence defining the education, background, and experience needed to meet minimum competency requirements for the role. In addition to defining these requirements, you would need to be able to demonstrate that the employees actually meet them on an ongoing basis.
If you have employees who perform highly specialized tasks that require expert training (e.g., CNC operator, welder, CPA, etc.), the personnel file should contain their certifications. Training in addition to education, background, and experience is a key part of establishing competence. However, no manager wants to train someone without an accounting background to become their Finance Director, and thats why establishing a written baseline for education, background, and experience is so important for each role. You can boost competence through training but cant turn a dandelion into a rose. Your job descriptions must clearly address the necessary education, background, and experience needed to perform the job. If you do not have well-defined job qualifications for each role and employee, you may want to first determine what education, background, and experience are necessary and then conduct a competence gap analysis.
Documenting the Training Every Employee Receives
FDA and Notified Bodies often cite companies for failing to document training. Heres an example of how seriously FDA views competence, training, and training records the excerpt below was taken from a published FDA warning letter, which we have shortened for clarity.
Failure to train production employees responsible for quality functions as required by 21 CFR 820.25(b): Your firm’s training procedurerequires all employees to be trained to perform the assigned responsibilities and also requires the maintenance of training records.However, no records were provided to demonstrate thatemployees who performed design control activities had been trained to the design control procedure.In fact, there were no training records at all for these employees. Your written response was reviewed and appears to be adequate, in that you have committed to training your research personnel with applicable QSIT principles.We also acknowledge that you may feel that thepersonnel who performed the design control activities were trained appropriately in research and innovation capabilities; however, quality assurance activities are not the same.If these employees had adequate experience as you have stated, we may not have discovered deficiencies related to the 510(k) submission which resulted in your firm voluntarily recalling product from the market.We would like to emphasize that employees performing functions within the realm of the Quality System Regulations for medical devices must be trained as such and that documentation of that training is a cGMP requirement.
Ouch.Most companies document training by making sure all employees sign the SOP after they have been trained on it. If you are holding a group training session, use a sign-in sheet with a line for each employee in attendance to sign. In terms of meeting FDA QSR 820.25(b) and ISO 13485:2016 training requirements, both are acceptable as documentation. Using the example discussed earlier, its not enough to email a copy of your ASL to an employee involved in purchasing and include a link to 21 CFR Part 820.50 (Purchasing Controls) on the FDA website with the expectation that the employee will study both. You must document that actual training occurred.
Most managers use a training matrix to help with the following:
- Identifying job-specific training requirements.
- Tracking completed training for individuals and departments.
- Demonstrating to an auditor or inspector that training is taking place and being tracked.
Example Training Record
Remember, the training matrix is not a replacement for individual training records. You cannot solely rely on a master Excel spreadsheet listing all employees on one worksheet and a check box next to Bob Smiths name saying he has been trained on SOP #24. The matrix should be used to organize your training effort, but you still need individual training records (or the equivalent through a learning management system) to prove that an employee has been trained.
Monitoring the Effectiveness of Your Training Efforts
A training matrix helps you track overall training programs, and training records prove that individual employees have been trained. But how do you know if the training has been effective’ Simple training records will not cut it you must assess the effectiveness of training. There are several ways to do this:
- Survey employees to see if they feel they have learned the information.
- Test them after the training and periodically thereafter.
- Evaluate their work performance to see if they are following SOPs and work instructions.
- Review the trainers assessment of effectiveness.
The importance of measuring effectiveness is driven home in ISO 13485:2016 with regard to risk management. It states: The risks should be considered if the given training is not fully understood. Consideration should specifically be given to what could be the consequences if employees interpret the essence of a certain training incorrectly and what the subsequent impact could be on a products quality.
Training Matrix Example

If you have a production line and there are specific employees responsible for doing inspections prior to shipment, you should obviously specifically train those employees on what to look for. But one way to reinforce effectiveness would be to add actual prop examples or posters with photos of various defects displayed near the inspection lines. This would provide constant visual reinforcement and an auditor would appreciate seeing this. Tip: Visual props and posters become invisible over time change them periodically and they will get noticed!
Another Way to Evaluate Training Programs
Many managers apply the well-known Kirkpatrick Model to measure training effectiveness. It consists of four levels.
We cannot stress enough that, in the eyes of regulators and auditors, monitoring of training effectiveness is really important more so than the training itself. As you know, training is not a one and done exercise: You dont simply hand a new employee a pile of SOPs and then hope for the best. Supervisors must (a) proactively check the employees work to make sure they are following specific SOPs, and (b) document the fact that this has been assessed. Keep in mind that annual performance evaluations cannot be used as evidence of training effectiveness in an audit.
Responses to corrective actions sometimes result in changes to procedures. Even if that change is updated in the SOP, managers sometimes forget (or are too busy) to retrain the employees on the change, or they do so informally but dont document it. FDA and Notified Bodies will often ding companies for this during audits, so make sure that departmental procedures include instructions to evaluate the impact of changes on relevant training.
Work with Oriel STAT A MATRIX to Bring Your Employee Training to the Next Level
Oriel STAT A MATRIX has been training medical device RA/QA professionals for more than 50 years. We have worked with more than 90% of the leading medical device companies to elevate the skills and competence of their employees. We can assist in many ways, from 300+ sessions of public RA/QA classes to on-site group training and coaching. Our team can also work with you assess training needs, map competencies to determine who requires specific training and when, establish benchmarks for assessing participants knowledge base, and design/execute certification programs to ensure employees obtain and gain expertise in the desired competencies. We then develop or update your RA/QA curriculum, and design and teach pilot courses to meet your needs. In this paradigm, your organization takes ownership of the program, but well be there to help or update the curriculum to adjust for changing regulations. Get in touch with us and we can tell you more!